From Biology to Technology
Synbio Consulting's Blog

SynBio Mashup #22
The SynBio Mashup is a weekly review of articles and news related to synthetic biology and metabolic engineering. While we share most of this on our twitter feed, if you need to catch up on this week’s news just read ahead!
Public consultation on the preliminary opinion on Synthetic Biology III: Research Priorities
Last week, the European Commission released to the general public a preliminary version of the third and final opinion on research priorities in synthetic biology. A consortium of three scientific committees and experts in the field, including those at Synbio Consulting, advised the Commission on issues relating to public health, consumer safety, and the environment. In the previous opinions, we defined synthetic biology to be “the application of science, technology and engineering to facilitate and accelerate the design, manufacture and/or modification of genetic materials in living organisms”.
The second opinion discussed whether the current regulations of the European Union for genetically modified organisms are adequate in regulating the risks for human and animal health and the environment and suggestions were provided for changes to the risk assessment procedures and risk mitigation procedures, including safety locks.
In this third opinion, major gaps in knowledge necessary for proper risk assessment are identified and research topics that would help close those gaps are suggested. This opinion is written to mitigate risks for the foreseeable future, but will need to be updated to include the social, ethical, governance, and security implications of synthetic biology as well as human embryonic research. The European Commission is currently seeking consultation from the public about this third opinion. If you have any comments, suggestions, explanations, contributions or any other scientific information that you feel the group should investigate, please submit your written comments here.
BioBuilder: Synthetic Biology in the Lab Now in Stores!
BioBuilder: Synthetic Biology in the Lab is a new book written by Natalie Kuldell, PhD., Rachel Bernstein, Kathryn M Hart and Illustrated by Karen Ingram. It is a wonderful introduction to synthetic biology. Developed at MIT in collaboration with award winning high school teachers, the book allows for both teachers and students to get the background information to get involved in the newly emerging synthetic biology field.
Based on BioBuilder’s curriculum, this book provides instruction for hands-on learning for secondary or post-secondary classrooms and laboratories. It teaches the fundamentals of synthetic biology, the key aspects that researchers are investigating in the lab, and ethical issues associated with synthetic biology. In addition, students will learn the “design, test, build cycle”, test synthetic organisms built in a lab, measure variants of an enzyme-generating genetic circuit, investigate “bacterial photography”, or even build living systems to generate a diversity of colorful pigments. Pick up a copy today!
Intrexon and Fibrocell Announce IND Filing for Treatment of Recessive Dystrophic Epidermolysis Bullosa (RDEB)
On July 20th, 2015, Intrexon and Fibrocell announced their filing of an Investigational New Drug (IND) Application for FCX-007 for the treatment of recessive dystrophic epidermolysis bullosa (RDEB). Intrexon, a leader in the synthetic biology industry, and Fibrocell, a company committed to the treatment of rare skin and connective tissue disorders, teamed up to create this innovative product for a disease that currently has no cure.
RDEB is a rare genetic connective tissue disorder, caused by a mutation in the COL7A1 gene that codes for type VII collagen, which forms anchoring fibrils between layers of the skin. This disease currently affects 1,100 – 2,500 Americans and causes skin layers to separate causing blistering, open wounds, and scarring during daily activities, such as rubbing or scratching and often leads to death. Children affected by this disease are often called “butterfly children” because their skin is as delicate as the wings of a butterfly.
FCX-007 is a novel gene therapy treatment for RDEB. It is a cultured genetically modified autologous fibroblast that encodes COL7, ex-vivo, which is then injected at local blister and wound sites. This allows for more effective and localized treatment of affected areas instead of systemic treatment. In pre-clinical studies, there were no findings of toxicology in RDEB human skin xenograft severe combined immunodeficiency (SCID) mice. Additionally, COL7 was found in dermal-epidermal junctions of RDEB cultured grafts in RDEB human skin xenograft SCID mice and there was no apparent systemic distribution of the vector in human skin xenograft SCID mice. These positive proofs of concept are allowing Intrexon and Fibrocell to initiate Phase I/II clinical trials by the end of this year.
Events:
4th Annual Sc2.0 & Synthetic Genomes Conference
On Thursday July 16th and Friday July 17th, 2015, the 4th Annual Sc2.0 & Synthetic Genomes Conference took place at New York Genome Center in New York City. The Synthetic Yeast Genome Project, Sc2.0, is attempting to generate an entirely synthetic genome for the yeast Saccharomyces cerevisiae. The conference discussed the collaborative effort of the Sc2.0 Research Consortium to generate 16 synthetic chromosomes of the designer genome. Genome engineering efforts, CRISPRs and designer nucleases and synthetic biology were also discussed. In addition, there was a panel discussion on genome engineering, industry and society, and keynote speakers from lab automation and DNA synthesis industries gave presentations. Look out for the next edition!
SynBioBeta Activate! Cambridge, UK 2015
On July 27th, 2015, SynBioBeta is holding their annual SynBioBeta Activate! conference in Cambridge, UK. It will be held at Old Divinity School, St. John’s College, Trinity Street, Cambridge, UK from 5-9:30pm. The topic of discussion is Reprogramming Life With Synthetic Biology.
There will be presentations from a number of leading companies in the synthetic biology industry and discussions around new tools and the open source innovation. The schedule is as follows:
5:30-6:00 – Arrival and Networking
6:00-6:30 – Synthetic Biology: New Tools for an Industry at an Inflection Point
6:30-7:15 – SynBio Company Showcase
7:15-7:45 – Panel: Can Open Source Biological Innovation Succeed?
7:45-9:00 – Drinks, Finger Buffet and Networking
Tickets are £5.00 for students and £10.00 for everyone else.
Register here!
That’s it for this week’s Synthetic Biology Mashup! A suggestion or a question? Shoot us an email!

Synbio Mashup #21
The Synthetic Biology Mashup is a weekly review of articles and news related to synthetic biology and metabolic engineering. While we share most of this on our twitter feed, if you need to catch up on this week’s news just read ahead!
Review of the Memorandum for Heads of the FDA, EPA, and USDA
On July 2, 2015, a memorandum was released for the heads of the United States Food and Drug Administration (FDA), Environmental Protection Agency (EPA), and the Department of Agriculture (USDA) to modernize the Federal regulatory system for biotech products and to establish periodic updates of the system. The purpose of this memorandum is to keep public confidence in the system high, allowing for the best novel scientific breakthroughs to be brought into the economy by preventing barriers to future innovation and development, improving coordination, transparency and efficiency throughout the industry, all while protecting human and animal health and the environment through risk assessment and regulation. The memorandum also sought to divide the responsibility of regulation across the three government agencies. The principals outlined in this memorandum have now been applied to the regulatory framework for the biotechnology industry, including Synthetic Biology.
Data Storage for Genetic Information
Stevens, et al. published an article on July 7, 2015 comparing future data storage of genomes to Big Data domains such as astronomy, Twitter, and Youtube. Genome sequencing is becoming more popular and less expensive and has implications in scientific discovery and personalized medicine. Currently the Sequence Read Archive (SRA) maintained by the United States National Institutes of Health (NIH) holds raw sequenced data of 32,000 microbial genomes, 5,000 plant genomes, and 250,000 individual human genomes. Although it seems to be a very high estimate, the authors of this article believe, 100 million to as many as 2 billion individual human genomes and 1.2 million plant and animal genomes for energy, environmental and agricultural purposes will be sequenced by the year 2025. The actual number of genomes sequenced will depend on a number of factors including decreasing the price of sequencing a genome (currently about $1,000 per genome), whether the computational technology to store the all of the data will be developed, willingness of the public to sequence their genomes, and government regulations preventing the sequencing of genomes outside of medical purposes. Additionally, it will be important to develop an adequate secure storage system, to prevent confidential medical information being released to the public. 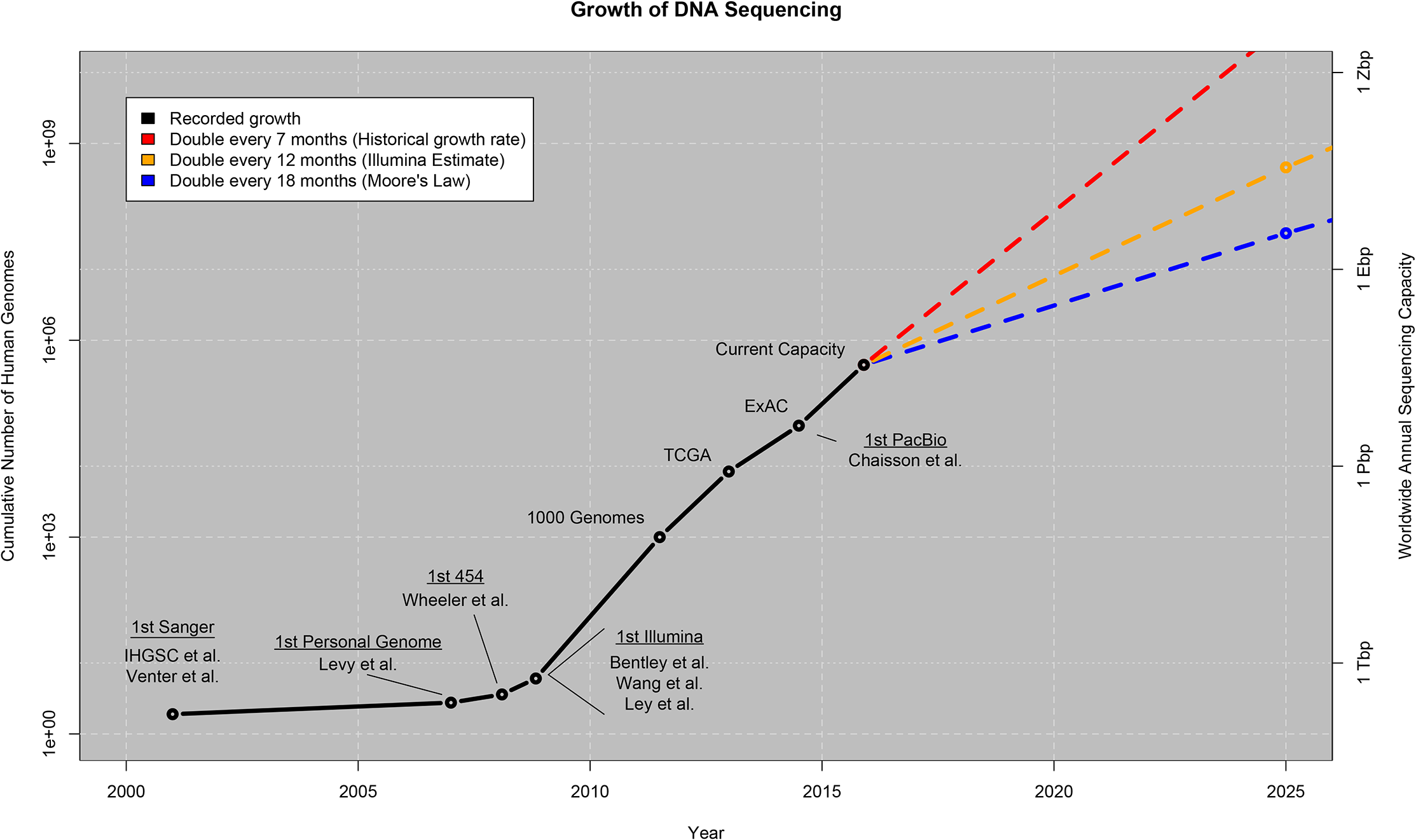 Figure 1: Projected growth of genomic sequencing technology. Source: Stephens, ZD., et al., Big Data: Astronomical or Genomical? PLoS Biology, 2015.
Figure 1: Projected growth of genomic sequencing technology. Source: Stephens, ZD., et al., Big Data: Astronomical or Genomical? PLoS Biology, 2015.
MIT Researchers Develop Computing Elements for Bacteria in the Gut
In a paper published in Cell on July 9, 2015, researchers at MIT modified basic computing elements in the bacterium Bacteroides thetaiotaomicron, a prevalent and abundant commensal bacterium of the human gut. These sensors, memory switches, and circuits are able to respond to signals in the gut and could potentially be used for for surveillance of or therapeutic delivery to the gut microbiome. Prior to this achivement, genetic circuits were only built inside of model organisms such as E. coli that is present only in low concentrations in the gut. Once an environmental stimulus, such as a food additive, has been detected by this genetically modified bacterium, genes can be turned on or off for controlled treatment of inflammation or cancerous tissue. The bacterium is also engineered to remember the environment by transcribing recombinases, which record information into bacterial DNA by recognizing specific DNA sequences and inverting their direction. Creating genetic circuits in commensal bacteria will potentially allow for non-invasive monitoring of the gut, long-term targeted therapeutics, and further functional studies of the digestive tract. If you would like to learn more, please read the published paper!
DEINOVE Partners with POS Bio-Sciences to Produce Carotenoids
DEINOVE, a French biotech company that uses Deinococcus radiodurans bacteria for the production of biofuels and bio-based chemicals recently partnered with Canada’s POS Bio-Sciences for the development, extraction and purification of carotenoids. POS Bio-Sciences is a leader in the extraction of high value compounds, such as carotenoids. D. radiodurans is an extremophile bacterium that can withstand large amounts of ionizing radiation, cold, dehydration, vacuum and acid. In recent years, DEINOVE’s DIENOCHEM division has been working to create a strain of D. radiodurans to increase its natural propensity to produce carotenoids. Humans and animals do not produce carotenoids de novo and carotenoids must be consumed from diet. Carotenoids prevent cancer, heart disease, inflammation, and vitamin A deficiency and promote eye health. They are also used in food coloring. The global market for carotenoids is expected to be $1.4 billion by 2019, with an estimated annual growth rate of 3.5%. To learn more, please read DEINOV’s press release!
New Biotech Funding
Eligo Bioscience
Led by the European venture capital firm, Seventure, Eligo Bioscience, a French startup hoping to revolutionize the antibiotics market, received a €2 million ($2,237,000) in Series A funding. Eligo Biosciences is a spin-off company from MIT and Rockefeller University. Their “Eligobiotics” technology harvests CRISPR-Cas nucleases to produce sequence-specific antimicrobials able to selectively target harmful bacteria, while leaving good bacteria unaffected. In addition to targeted therapy, the “antibiotic” induces environmental pressure and competition between the good and bad bacteria to stop the infection. Eligo Bioscience technology has potential applications to treat infections in the skin, mouth, gut and vaginal microbiomes.
Clara Foods
Clara Foods, a spin-off of the artificial meat production company New Harvest, recently closed a deal worth $1.7 million in seed funding for their project to generate egg whites from genetically modified yeast instead of chickens. If Clara Foods can scale up production, their product could significantly decrease the cost producing egg whites. They could feasibly market to companies that use a large amount of egg whites in their production, such as pasta and condiment companies. Clara Foods uses similar synthetic biology technology to Hampton Creek, a plant based food producer, and Muufri, an animal free milk producer. In addition to decreasing the cost of egg whites, their product decreases the environmental impact of raising chickens and avoids the transmission of egg-borne diseases, such as salmonella and the avian-flu, while maintaining the same nutritional value as traditional eggs. Clara Foods is working with IndieBio to expand their business and connect with customers and investors. The company is currently trying to generate cooperate partners, which means that lab-grown eggs may be in the foods you eat sooner than you think!
Twist Biosciences
Twist Biosciences, a company pioneering breakthrough DNA synthesis technologies announced on June 9, 2015 that they closed a deal led by Illumina, Inc. for $37 million in Series C funding. To this date, Twist Biosciences has raised $82.1 million in funding. The funding will be used for a beta commercial launch their silicon-based DNA synthesis platform. Because silicon is a better conductor of heat , Twist Biosciences ~10,000 well silicon technology accelerates DNA synthesis reactions by ~100x compared to traditional plastic 96-well plates. They will be able to create oligionucleotides, genes, pathways and genomes at a faster rate and a cheaper cost. This can have major implications in synthetic biology industries such as personalized medicine and in vivo diagnostics, sustainable chemical production, agriculture, biodetection and data.
Events Not To Miss!
Genspace – Introduction to Synthetic Biology
On Wednesday July 15, 2015 Genspace [D1] NYC in Brooklyn, New York, is hosting a special event. Synthetic Biologist and professor Dr. Natalie Kuldell and designer Karen Ingram will discuss their collaboration on their new book “BioBuilder: Synthetic Biology in the Lab”. [D2] In addition Professor Paul Freedman from the Imperial College of London will discuss the latest developments in the Synthetic Biology field. Doors open at 6:30pm for wine and cheese and the talks begin at 7pm. If you are interested in attending, RSVP here!
Lunch Seminar On Genome Engineering for Xenotransplantation
On July 20th, 2015, Sean Stevens, mammalian synthetic biologists who works on synthetic genomes, will be giving a lunch seminar on genome engineering for xenotransplantation. Xenotransplantation is the transplantation of organs, tissues and cells between species. Once the immunological barriers have been overcome, this technology can potentially be used to transplant organs from pigs to humans. If you are interested in learning more, come to the seminar at South San Francisco Conference Center, 255 S Airport Blvd, South San Francisco, CA 94080 from 11:30am to 1:30pm. Register for free here!

Training the Next Generation of Leaders in Synthetic Biology
A Small Overview of a Piece We Recently Published in Science Careers
Training the next generation of leaders for the bio-industry is a great challenge. Most graduate programs are still geared towards tenure track and do not reflect the reality of the work place. Data published in the National Science Board’s 2014 Science and Engineering Indicators show that a mere 29% of newly graduated life science PhDs will find a full time faculty position.
With our colleague, Dr. Jim Philp from the Organization for Economic Co-operation and Development, we wrote a small piece for Science Careers to assess the situation and give pointers to better train the next generation of leaders in the Synthetic Biology industry. We provide here a few takeaways, but make sure to read the full article on Science Careers.
As defined by the European Commission, Synthetic Biology is “the application of science, technology and engineering to facilitate and accelerate the design, manufacture and/or modification of genetic materials in living organisms.” Although the field is in its infancy stage, it has been estimated that the global market for synthetic biology will reach $39 billion by 2020 in industries such as climate change, energy consumption, environmental protection, and health care. Facing such a potential, educational institutions ought to adapt and propose a diverse range of programs spanning not only basic science but also entrepreneurship and management.
A great getaway for students to get involved in the synthetic biology industry is to compete in an international synthetic biology competition such as the International Genetically Engineered Machine competition (iGEM). There are also more than 100 universities now involved in educating future synthetic biologists at the Masters and Ph.D. levels. Scientists interested in the field usually have backgrounds in mathematics, physics, computer sciences, chemistry, biology, and medicine. This diversity of backgrounds and inherent interdisciplinary nature of the field makes for interesting educational challenges.
Beyond academic programs, navigating and understanding and capitalizing on the exciting options to transition into business and entrepreneurship might prove overwhelming. There are no MBAs specific for the synthetic biology industry, MBAs geared towards the biotechnology and chemical industries may be a good starting. Beyond this the international Synthetic Biology Leadership Accelerator Program (LEAP) can help synthetic biology change-makers and support them to positively impact the field. Last but not least, a number of startup accelerator such as Indie.Bio are starting to provide financial support and mentorship to early-stage synthetic biology startups.
Make sure to read the full article on Science Careers and let us know if you have any questions! Info@Synbioconsulting.com.
